- All
- Product Management
- News
- Introduction
- Enterprise outlets
- FAQ
- Enterprise Video
- Enterprise Atlas
The emergence of 'post-biotics' injects new momentum into the development of the health industry.
Postbiotics is a new concept that has emerged in the past two years. For many people, it is still somewhat unfamiliar, but once it was proposed, it attracted widespread attention from industries such as food and dietary supplements, and its popularity has been increasing day by day. It is a new trend in the industry, and research on it is becoming more in-depth.
In May 2021, the International Scientific Association for Probiotics and Prebiotics (ISAPP) released a consensus statement on postbiotics, officially making postbiotics a category of great interest in the global gut microbiome field.
2021 was marked asthe 'Year of Postbiotics'.In August 2022, the Probiotics Branch of the Chinese Institute of Food Science and Technology officially released 'The Research Status and Industrial Application of Postbiotics', which pointed out the direction for the scientific research and industrial development of 'postbiotics'.In January 2023, China's group standard for postbiotics, 'Probiotic Products Lactobacillus Postbiotics', was officially released. As the first group standard in the field of postbiotics in China, the release of this standard has epoch-making significance for the high-quality development of China's probiotics and postbiotics industry, marking the beginning of a regulated development path for the postbiotics industry in China.
Chuangyuan Biotechnology, as one of the main drafting units of the postbiotics group standard, participated in empowering innovation for the industry.
The group standard defines postbiotics as: inactivated microorganisms and/or microbial components with a clear genetic background that are beneficial to host health, including or excluding their metabolites; excluding chemically synthesized components and viruses/bacteriophages and their products.It also emphasizes the raw material requirements for postbiotic products, stating that the strains should meet four major conditions:
(1) They should comply with the 'List of Microbial Strains Approved for Food Use' and the announced regulations issued by the state;
(2) The strain number and isolation source should be clearly defined;
(3) Identification and safety evaluation should be conducted at the strain level by qualified institutions using national or internationally recognized evaluation methods;
(4) Whole genome sequencing should be performed, and corresponding scientific literature or reports should be provided.
Advantages of postbiotics compared to probiotics
Higher safety:

They do not require low-temperature storage or cold chain transportation, have a wide range of storage temperatures, and therefore have a longer shelf life. Additionally, the effective microbial components, such as lipopolysaccharides, contain a high ratio of proline, which protects them from damage by acids, bases, and heat, resulting in better tolerance.Not affected by antibiotics:
Postbiotics are not inhibited by antibiotics, while probiotics are difficult to use simultaneously with antibiotics and carry the risk of transmitting antibiotic resistance genes.Conducive to maintaining host balance:
They support the production of more probiotics in the human digestive system.No need to consider the interference of gastrointestinal fluids:
There is no need to consider the digestive degradation effects of human gastrointestinal fluids, and they do not need to colonize to exert their effects.Wide application range:
Due to their stability across a wide range of temperatures and pH levels, they can be added to high-acid foods before thermal processing and can be widely used in various products: juices, beverages, dairy products, candies, milk powder, cereals, and other general foods that require processing.Postbiotics & related regulations and standards for food
Currently, there are no clear standards and regulations specifically for postbiotics released domestically or internationally, nor have regulatory agencies proposed definitions for foods or dietary supplements containing postbiotics. However, some food product management requirements in various countries align with the definition of postbiotics. In the medical and pharmaceutical fields, regulatory requirements for the application of postbiotics have been introduced, such as in 2019, when the European Union released relevant documents for a bacterial lysate drug used to prevent recurrent respiratory infections.China:
The U.S. Food and Drug Administration (FDA) manages food strains and food additives through the GRAS system, using a 'self-affirmation by the company, FDA filing system' to evaluate the safety of substances and form a GRAS list. Currently, the FDA allows the application of inactivated strains in food to be filed as GRAS.
Canada:In the 'Natural Health Product Regulations (NHPR) SOR/2003-196' released by the Department of Justice, bacteria, fungi, and their extracts or isolates with unchanged molecular structures can be considered 'natural health products' (NHP). Based on this, some inactivated microbial products can be submitted to Health Canada for approval as natural health products, with the department reviewing the product's ingredients and health effects.
European Union:The European Food Safety Authority assesses the safety of food strains through the QPS procedure, which can be applied to evaluate the microorganisms used to prepare postbiotics. If microorganisms not listed in the QPS list are used to produce food, they must be declared for new resource food approval according to the relevant requirements of the EU Novel Food Regulation (EU) 2015/2283.
Japan:司法部发布的《天然健康食品条例(Natural Health Product Regulations (NHPR)SOR/2003-196)》中,细菌、真菌及其分子结构未改变的提取物或分离物均可视为“天然健康食品”(NHP)。基于此,部分灭活微生物产品可向加拿大卫生部提交申请作为天然健康食品,由卫生部审查产品成分及健康作用。
欧盟:欧盟食品安全局通过QPS程序评估食品用菌种的安全性,可适用于评估制备后生元的微生物。若使用未列入QPS名单的微生物生产食品,需根据欧盟新资源食品管理条例 Regulation(EU)2015/2283相关要求申报新资源食品审批。
日本:There are three regulatory channels to apply for health claims on food: Foods for Specified Health Uses (FOSHU), Foods with Nutritional Function Claims (FNFC), and Foods with Function Claims (FFC). Currently, no products containing inactivated microorganisms have been approved as FOSHU. FFC does not require approval, but necessary materials such as safety, efficacy evaluation, scientific basis, and production and quality management methods must be submitted to the consumer department 60 days before market launch, and the government does not provide a license label. Currently, only a few inactivated lactic acid bacteria products have been granted FFC approval.
Oceania:The Food Standards Australia New Zealand (FSANZ) and the Australian Department of Health have not yet issued standards and regulations related to postbiotics. The 'Food Standards Code' states that if a food needs to declare health claims, the company must conduct a systematic review to confirm the relationship between the food and the health claim and inform the Australian government of the evaluation results.
Although it started later, with increasing academic attention on postbiotics, it has now become a new trend in food.
Driven by the rising health awareness among residents and the increasing demand for health foods, major global food companies are committed to the research and development of postbiotic-related products, especially in developed regions such as Japan, North America, and Europe, where the postbiotic industry chain is taking shape and the commercialization process is continuously advancing.
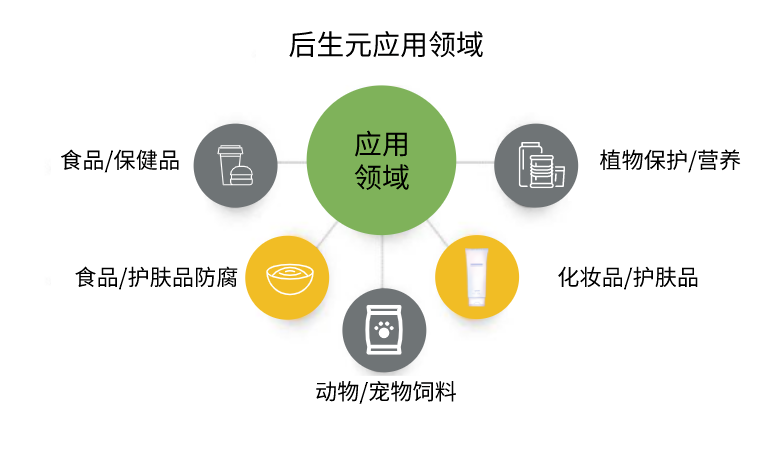
Postbiotics have been applied in various fields including regular food, health food, special medical purpose formula food, and infant food.
In the food sector, postbiotics have numerous recognized and emerging food safety functions in areas such as food biopreservation and packaging, biodegradation of harmful chemical pollutants (such as pesticides, mycotoxins, and bile acids), and control of foodborne pathogen biofilms. They can be widely applied in various food categories, such as beverages, dairy products, cereals, biscuits, bread, and cakes.
The effectiveness of postbiotics in food matrices depends on the probiotic strains that derive the postbiotics, the type of target microorganisms or contaminants, the concentration and form (liquid/powder) used, and the characteristics of the food matrix. The application of postbiotics in food meets consumer health demands to some extent and has broad development prospects.
Currently, postbiotic products in the international market are presented in a rich variety of forms. Many companies have successively launched postbiotic concept products, including nutritional supplements, lactic acid bacteria beverages, tofu, baked goods, and snacks, bringing this concept into daily diets.
However, there are still many challenges in the research and development of postbiotics or probiotic derivatives. For example, the exact mechanisms behind their special roles in certain systems or diseases, as well as the dose-response relationship of their efficacy when added to food matrices, are not yet fully clarified; how to qualitatively and quantitatively characterize the product quality of foods containing postbiotics; and there is still a lack of new processes and technologies for large-scale industrial production of postbiotics.
With the continuous optimization of the definition of postbiotics, consumers are gradually paying attention to this concept. According to a survey by Mintel, the attention of global internet users to postbiotics on social media in 2019 was nearly double that of 2018; the relevant search volume on search engines also increased more than tenfold between 2019 and 2021. In terms of research, data from the literature website PubMed Central (PMC) shows that research on postbiotics has significantly increased in recent years, with nearly 200 papers published in 2020.
In recent years, functional foods containing probiotics and prebiotics have attracted widespread attention from brands and consumers. The market already has a variety of foods containing bioactive substances to meet the nutritional needs of customers with different dietary preferences. Compared to probiotics and prebiotics, postbiotics have unique advantages, offering more flexibility in product category innovation, broadening formulation choices, and better achieving certain product forms and manufacturing conditions.

Related News


